Harness the Power of Knowledge
Regulatory research serves as the backbone of the healthcare industry, playing an important role in making sure that all novel drugs, medical devices, and therapeutic strategies conform to established safety and efficacy benchmarks. This sector revolves around a comprehensive understanding and meticulous application of the laws, regulations, guidelines, and processes that govern the development, manufacture, and marketing of healthcare products.
The significance of regulatory research in the healthcare industry is amplified particularly in the context of the fast-paced evolution of genomics. It’s 2023, and the field of genomics is hot with groundbreaking trends such as Next Generation Sequencing (NGS), Genome Data Analysis, Genome Editing, Artificial Intelligence, Functional Genomics, Meta-genomics, Single-Cell Genomics, and 3D Genomics and so much more. These trends, sparked by biotech startups and big pharma, are revolutionizing our perception of biological science, and enhancing our capabilities to improve human health.
However, such rapid progress brings with it many regulatory challenges. Regulatory research needs to match the pace of genomic technology evolution and ensure that these advancements are harnessed within a framework that champions safety, ethics, and efficacy. If you are interested in getting into the regulatory research field, you found the right place.
Your Journey Through a Career in Regulatory Research
A career in regulatory research often starts with a robust foundation in life sciences, supplemented by specialized education in regulatory affairs. This professional journey can open doors to diverse roles in the pharmaceutical, biotech, and medical device industries, in government bodies like the FDA, and in regulatory consulting firms.
The journey through a career in regulatory research means grappling with the regulatory implications of new technological breakthroughs. Regulatory affairs professionals are entrusted with understanding these novel technologies, interpreting and implementing the relevant regulations, and striking a balance between fostering innovation and safeguarding public health.
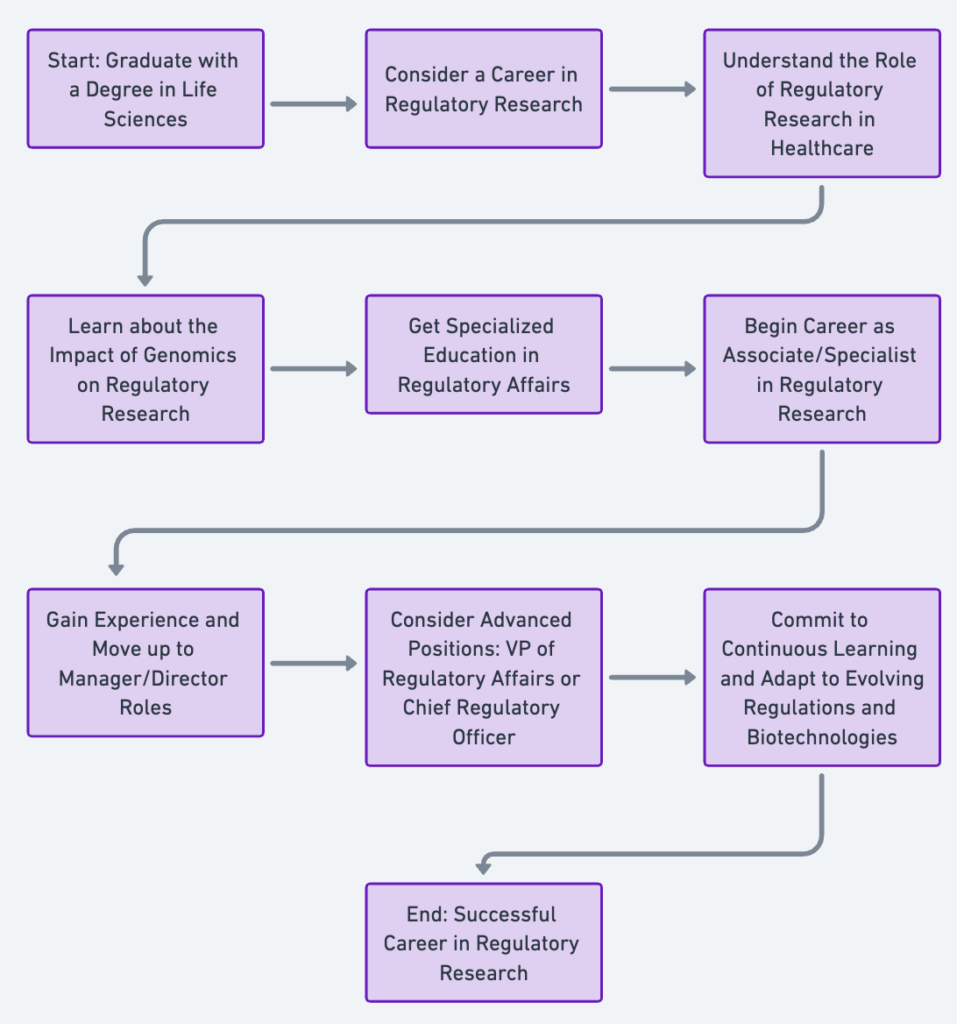
A career in regulatory research within the biotech industry is a journey that involves the intersection of science, policy, and law. The typical career path in this field often begins with a strong foundation in life sciences. Many regulatory research professionals hold degrees in biology, biochemistry, pharmacology, or a similar field. This foundational knowledge is critical because it provides an understanding of the principles of science, which is essential for evaluating the safety, efficacy, and quality of biotechnological products.
Upon obtaining a degree, individuals often start their career in regulatory research as an associate or a specialist. This is a hands-on role, working directly with the development and testing of new biotech products, ensuring they meet regulatory guidelines and standards. It also involves preparing and submitting documents to regulatory authorities like the FDA, EMA, or other global equivalents.
After gaining experience, professionals often move up to become regulatory affairs managers or directors. In these roles, they are responsible for strategic planning and decision-making, ensuring their company’s products are compliant with all the necessary regulations in the markets they operate in. They liaise with regulatory authorities, oversee product development teams, provide strategic input into clinical development, and work on post-market surveillance, ensuring legal and regulatory compliance.
Advanced positions in regulatory research might include becoming a Vice President of Regulatory Affairs or a Chief Regulatory Officer. These are highly strategic roles, often involving the oversight of regulatory affairs across multiple products and geographies, and serving as the primary interface with regulatory bodies on a corporate level.
Continuous learning is a fundamental aspect of progression in this field, as regulations and biotechnologies are always evolving. Many professionals also earn additional certifications or degrees in regulatory affairs or law to deepen their knowledge and advance their careers.
Exploring Educational Pathways and Certifications for Regulatory Research
A career in regulatory research generally necessitates an undergraduate degree in a life science discipline, complemented by a master’s degree or certification in regulatory affairs. Various universities and institutions, often in partnership with industry stakeholders, provide these specialized programs.
With the surge of genomics in healthcare and the swift pace of progress in this sector, regulatory affairs professionals are encouraged to consider further education or training in genomics and bioinformatics. Such expertise could be indispensable in deciphering the intricacies of emerging technologies and assessing their potential societal impacts.
By grasping the scientific principles underpinning these technologies, regulatory professionals can make more informed decisions and play a significant role in the formulation and implementation of regulations that steer the use of these promising new technologies in healthcare.
For those seeking educational pathways and certifications for a career in regulatory research, there are several options available listed below:
Degree Programs in Regulatory Affairs:
- Master’s in Regulatory Science at Arizona State University (ASU), preparing individuals to ensure the safety of medical research, pharmaceuticals, and medical devices.
- MS in Regulatory Science at Johns Hopkins University, focusing on compliance, consumer safety, and public health.
- Master of Science in Regulatory Affairs at Northeastern University, designed to manage the global regulatory process for companies innovating and developing cutting-edge products in healthcare and food safety.
- The University of Southern California (USC) offers a Master of Science in Regulatory Research.
- Master of Regulatory Affairs degree program offered by the University of Pennsylvania, an institution of higher education authorized to confer degrees and certificates conferring academic credit under applicable laws of the United States.
- Regulatory Affairs and Quality Assurance program at Temple University School of Pharmacy, one of the oldest and most recognized programs of its kind.
Certificate Programs in Regulatory Affairs:

- The George Washington University online Graduate Certificate in Regulatory Affairs, is designed to teach working professionals the relevant, critical skills and knowledge they need to become regulatory affairs leaders in drug development.
- Regulatory Affairs Certificate Program by RAPS, an online series of courses personalized to meet your professional development needs.
- The Regulatory Affairs certificate program by UCSC Silicon Valley Extension, is aimed at developing a global regulatory strategy for medical devices and/or drugs and biologics.
- Regulatory Affairs Graduate Certificate at MCPHS, a flexible three-course certificate program building on your bachelor’s degree in related areas such as pharmacy, business administration, nursing, marketing, and public health.
- Certificate in Biomedical Regulatory Affairs by the University of Washington, focusing on the roles and responsibilities of regulatory affairs professionals in the development of new medical products.
- Regulatory Affairs and Compliance program by the University of California, Irvine, designed to help meet the expanding need for regulatory affairs professionals who are able to understand and interpret regulations across the full spectrum.
Certificate Programs in Genomics and Bioinformatics:
- Python for Genomic Data Science course offered by Johns Hopkins University on Coursera, teaching Python programming, statistical programming, data analysis, and bioinformatics among other skills.
- Bioinformatics Graduate Certificate at Harvard Extension School, focused on identifying, compiling, analyzing, and communicating complex biological and genetic data.
- Post-Master’s Graduate Certificate in Sequence Analysis and Genomics at Johns Hopkins University
Envisioning the Future of Regulatory Research
The future of regulatory research is intrinsically linked to the rapid advancements in medical technology and personalized medicine. As we delve deeper into the complexities of the human body at the molecular level, regulatory professionals will be instrumental in ensuring these discoveries are transformed into safe and effective therapies.
A career in regulatory research provides a unique platform to contribute to the development of state-of-the-art medical therapies. By keeping a finger on the pulse of the latest advancements in genomics and related fields, regulatory research professionals can help sculpt the future of healthcare. You can be better prepared by staying up to date at Greatness.bio.